Dosing and Administration
| Dosage Forms1 | Recommended Dose |
|---|---|
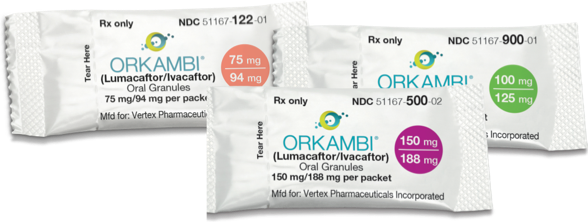
Oral Granules Not actual size. |
Age 1 through 2 years
Age 2 through 5 years
|
Tablets Not actual size. |
For patients age 6 years and older
|
| Dosage Forms1 |
Oral Granules Not actual size. |
| Recommended Dose |
Age 1 through 2 years
Age 2 through 5 years
|
| Dosage Forms1 |
Tablets Not actual size. |
| Recommended Dose |
For patients age 6 years and older
|
a7 to 9 kg ≈ 15 to 20 lb.
b9 to 14 kg ≈ 20 to 31 lb.
c14 kg ≈ 31 lb.
Note: A safe and efficacious dose of ORKAMBI for patients younger than 1 year has not been established. The use of ORKAMBI (oral granules) in children younger than 1 year is not recommended.
- ORKAMBI oral granules and tablets should be taken with fat-containing food1
- Advise patients to avoid grapefruit products during the first week after treatment initiation with ORKAMBI1
- Patients should continue taking all their prescribed CF therapies with ORKAMBI1
DOSAGE ADJUSTMENTS FOR ORKAMBI
| Tablet Dose¹ | Oral Granules Dose¹ | |||
|---|---|---|---|---|
| Hepatic Impairment | ||||
|
Severe impairment |
1 tablet in the morning and 1 tablet in the evening or less frequently | 1 packet in the morning or less frequently. No dose in the evening | ||
|
Moderate impairment |
2 tablets in the morning and 1 tablet in the evening for patients age 6 years and older | 1 packet in the morning every day and 1 packet in the evening every other day for patients age 1 to 5 years | ||
|
Mild impairment |
No dose adjustment required | |||
| CYP3A Inhibitorse | ||||
|
Initiating ORKAMBI in patients already taking a strong CYP3A inhibitor (eg, itraconazole)f |
First Week | After First Week | First Week | After First Week |
| 1 tablet daily | Continue with the full recommended daily dose as prescribed | 1 packet every other day | Continue with the full recommended daily dose as prescribed | |
|
Initiating CYP3A inhibitors in patients already taking ORKAMBIg |
No dose adjustment required | |||
|
Dose interruptions of ORKAMBI while taking strong CY3PA inhibitors |
If ORKAMBI is interrupted for more than 1 week and then reinitiated while taking strong CYP3A inhibitors, reduce dose to 1 tablet daily or 1 packet every other day for the first week of treatment reinitiation. Following this period, continue with the full recommended daily dose as prescribed. | |||
| Hepatic Impairment | ||
| Tablet Dose1 | ||
|
Severe impairment |
1 tablet in the morning and 1 tablet in the evening or less frequently | |
|
Moderate impairment |
2 tablets in the morning and 1 tablet |
|
|
Mild impairment |
No dose adjustment required | |
|
Oral Granules Dose1 |
||
|
Severe impairment |
1 packet in the morning or less frequently. No dose in the evening |
|
|
Moderate impairment |
1 packet in the morning every day and 1 packet in the evening every other day for patients age 1 to 5 years |
|
|
Mild impairment |
No dose adjustment required | |
| CYP3A Inhibitorse | ||
| Tablet Dose1 | ||
|
Initiating ORKAMBI in patients already taking a strong CYP3A inhibitor (eg, itraconazole)f |
First Week | After First Week |
|
1 tablet daily
|
Continue with the full recommended daily dose as prescribed |
|
|
Initiating CYP3A inhibitors in patients already taking ORKAMBIg |
No dose adjustment required |
|
|
Dose interruptions of ORKAMBI while taking strong CY3PA inhibitors |
If ORKAMBI is interrupted for more than 1 week and then reinitiated while taking strong CYP3A inhibitors, reduce dose to 1 tablet daily or 1 packet every other day for the first week of treatment reinitiation. Following this period, continue with the full recommended daily dose as prescribed. |
|
|
Oral Granules Dose1 |
||
|
Initiating ORKAMBI in patients already taking a strong CYP3A inhibitor (eg, itraconazole)f |
First Week | After First Week |
|
1 tablet daily
|
Continue with the full recommended daily dose as prescribed |
|
|
Initiating CYP3A inhibitors in patients already taking ORKAMBIg |
No dose adjustment required |
|
|
Dose interruptions of ORKAMBI while taking strong CY3PA inhibitors |
If ORKAMBI is interrupted for more than 1 week and then reinitiated while taking strong CYP3A inhibitors, reduce dose to 1 tablet daily or 1 packet every other day for the first week of treatment reinitiation. Following this period, continue with the full recommended daily dose as prescribed. |
|
dUse with caution after weighing the risks and benefits of treatment.1
eAdvise patients to avoid grapefruit products during the first week after treatment initiation with ORKAMBI.1
fAdditional examples include ketoconazole, posaconazole, voriconazole, telithromycin, and clarithromycin.1
gNo dose adjustment is recommended when used with moderate or weak CYP3A inhibitors.1
MISSED DOSE¹
- If ≤6 hours have passed: Advise patient to take the dose with fat-containing food
- If >6 hours have passed: Advise patient to skip that dose and resume the normal schedule for the following dose. A double dose should not be taken to make up for the forgotten dose
USE OF ORKAMBI IN SPECIFIC PATIENT POPULATIONS
Pregnancy1
- There are limited and incomplete human data from clinical trials and postmarketing reports on use of ORKAMBI or its individual components, lumacaftor or ivacaftor, in pregnant women to inform a drug-associated risk
Lactation1
- There is no information regarding the presence of lumacaftor or ivacaftor in human milk, the effects on the breastfed infant, or the effects on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for ORKAMBI and any potential adverse effects on the breastfed child from ORKAMBI or from the underlying maternal condition
Females and Males of Reproductive Potential1
- ORKAMBI may decrease hormonal contraceptive exposure, reducing the effectiveness. Hormonal contraceptives, including oral, injectable, transdermal, and implantable, should not be relied upon as an effective method of contraception when co-administered with ORKAMBI
Pediatric Use1
- The safety and efficacy of ORKAMBI in patients with CF younger than 1 year of age have not been established. Cases of non-congenital lens opacities have been reported in pediatric patients treated with ORKAMBI and ivacaftor, a component of ORKAMBI. Although other risk factors were present in some cases (such as corticosteroid use and exposure to radiation), a possible risk attributable to ivacaftor cannot be excluded
Geriatric Use1
- Clinical trials of ORKAMBI did not include sufficient numbers of patients 65 years of age and over to determine whether they respond differently from younger patients
Hepatic Impairment1
- No dose adjustment is necessary for patients with mild hepatic impairment (Child-Pugh Class A). A dose reduction is recommended for patients with moderate hepatic impairment (Child-Pugh Class B). Studies have not been conducted in patients with severe hepatic impairment (Child-Pugh Class C), but exposure is expected to be higher than in patients with moderate hepatic impairment. A dose reduction is recommended for patients with severe hepatic impairment. Use with caution in patients with severe hepatic impairment after weighing the risks and benefits of treatment. See dose chart above for recommended dose adjustments
Renal Impairment1
- ORKAMBI has not been studied in patients with mild, moderate, or severe renal impairment or in patients with end-stage renal disease
- No dose adjustment is necessary for patients with mild to moderate renal impairment. Caution is recommended while using ORKAMBI in patients with severe renal impairment (creatinine clearance ≤30 mL/min) or end-stage renal disease
| Degree of Renal Impairment | ORKAMBI |
|---|---|
| Mild to Moderate | No dosage adjustment |
| Severe (creatine clearance ≤30 mL/min) | Caution is recommended |
| End-Stage Renal Disease | Caution is recommended |
Patients With Severe Lung Dysfunction1
- The Phase 3 trials (Trials 1 and 2) included 29 patients receiving ORKAMBI with percent predicted FEV1 (ppFEV1) <40 at baseline. The treatment effect in this subgroup was comparable to that observed in patients with ppFEV1 ≥40.
- Respiratory events (eg, chest discomfort, dyspnea, and respiration abnormal) were observed more commonly in patients during initiation of ORKAMBI compared to those who received placebo. These events have led to drug discontinuation and can be serious, particularly in patients with advanced lung disease (ppFEV1 <40). Clinical experience in patients with ppFEV1 <40 is limited, and additional monitoring of these patients is recommended during initiation of therapy
Patients After Organ Transplantation1
- ORKAMBI has not been studied in patients with CF who have undergone organ transplantation. Use in transplanted patients is not recommended due to potential drug-drug interactions
ADMINISTRATION
Oral Granules: Preparation and Administration1
- Hold the packet with cut line on top
- Shake the packet gently to settle the ORKAMBI granules
- Tear or cut packet open along the line
- Carefully pour all of the ORKAMBI granules in the packet into 1 teaspoon of soft food or liquid in a small container
- Food or liquid should be at or below room temperature
- After mixing, caregiver should give within 1 hour
- It is important that patients consume the entire oral granules mixture with each dose
 Not actual size.
Not actual size. - Examples of soft foods or liquids include: puréed fruits, applesauce, flavored yogurt, pudding, milk, or juice
Tablets: Administration1
- Take 2 tablets twice a day, 12 hours apart
 Not actual size.
Not actual size.
Give Oral Granules and Tablets with Fat-Containing Food1
- For patients taking oral granules mixed into a soft food or liquid: dose must be taken just before or after consuming fat-containing food
- For patients taking tablets: dose must be taken with fat-containing food
| Examples of Fat-Containing Foods1 | ||
|---|---|---|
|
|
|
Palatability of ORKAMBI Oral Granules2,3
- Children may find the taste of the oral granules to be bitter
- Mixing the oral granules with soft foods or liquids that are sweet or rich, such as pudding or chocolate sauce, may help with the taste
For more information for caregivers, including tips for taking, please see the Downloadable Materials page.
ppFEV1, percent predicted forced expiratory volume in 1 second.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Use in Patients With Advanced Liver Disease
- Worsening of liver function, including hepatic encephalopathy, in patients with advanced liver disease has been reported. Liver function decompensation, including liver failure leading to death, has been reported in CF patients with pre-existing cirrhosis with portal hypertension while receiving ORKAMBI. Use ORKAMBI with caution in patients with advanced liver disease and only if the benefits are expected to outweigh the risks. If ORKAMBI is used in these patients, they should be closely monitored after the initiation of treatment and the dose should be reduced
INDICATION AND USAGE
ORKAMBI is a combination of lumacaftor and ivacaftor indicated for the treatment of cystic fibrosis (CF) in patients aged 1 year and older who are homozygous for the F508del mutation in the CFTR gene. If the patient's genotype is unknown, an FDA-cleared CF mutation test should be used to detect the presence of the F508del mutation on both alleles of the CFTR gene.
Limitations of Use
The efficacy and safety of ORKAMBI have not been established in patients with CF other than those homozygous for the F508del mutation.
INDICATION AND USAGE
ORKAMBI is a combination of lumacaftor and ivacaftor indicated for the treatment of cystic fibrosis (CF) in patients aged 1 year and older who are homozygous for the F508del mutation in the CFTR gene. If the patient's genotype is unknown, an FDA-cleared CF mutation test should be used to detect the presence of the F508del mutation on both alleles of the CFTR gene.
Limitations of Use
The efficacy and safety of ORKAMBI have not been established in patients with CF other than those homozygous for the F508del mutation.
IMPORTANT SAFETY INFORMATION
WARNINGS AND PRECAUTIONS
Use in Patients With Advanced Liver Disease
- Worsening of liver function, including hepatic encephalopathy, in patients with advanced liver disease has been reported. Liver function decompensation, including liver failure leading to death, has been reported in CF patients with pre-existing cirrhosis with portal hypertension while receiving ORKAMBI. Use ORKAMBI with caution in patients with advanced liver disease and only if the benefits are expected to outweigh the risks. If ORKAMBI is used in these patients, they should be closely monitored after the initiation of treatment and the dose should be reduced
Liver-related Events
- Serious adverse reactions related to elevated transaminases have been reported in patients with CF receiving ORKAMBI. In some instances, these elevations have been associated with concomitant elevations in total serum bilirubin
- It is recommended that ALT, AST, and bilirubin be assessed prior to initiating ORKAMBI, every 3 months during the first year of treatment, and annually thereafter. For patients with a history of ALT, AST, or bilirubin elevations, more frequent monitoring should be considered. Patients who develop increased ALT, AST, or bilirubin should be closely monitored until the abnormalities resolve
- Dosing should be interrupted in patients with ALT or AST >5 x upper limit of normal (ULN) when not associated with elevated bilirubin. Dosing should also be interrupted in patients with ALT or AST elevations >3 x ULN when associated with bilirubin elevations >2 x ULN. Following resolution of transaminase elevations, consider the benefits and risks of resuming dosing
Hypersensitivity Reactions, Including Anaphylaxis
- Hypersensitivity reactions, including cases of angioedema and anaphylaxis, have been reported in the postmarketing setting. If signs or symptoms of serious hypersensitivity reactions develop during treatment, discontinue ORKAMBI and institute appropriate therapy. Consider the benefits and risks for the individual patient to determine whether to resume treatment with ORKAMBI
Respiratory Events
- Respiratory events (e.g., chest discomfort, dyspnea, and respiration abnormal) were observed more commonly in patients during initiation of ORKAMBI compared to those who received placebo. These events have led to drug discontinuation and can be serious, particularly in patients with advanced lung disease (percent predicted FEV1 (ppFEV1) <40). Clinical experience in patients with ppFEV1 <40 is limited, and additional monitoring of these patients is recommended during initiation of therapy
Effect on Blood Pressure
- Increased blood pressure has been observed in some patients treated with ORKAMBI. Blood pressure should be monitored periodically in all patients being treated with ORKAMBI
Drug Interactions
Substrates of CYP3A
- Lumacaftor is a strong inducer of CYP3A. Administration of ORKAMBI may decrease systemic exposure of medicinal products that are substrates of CYP3A, which may decrease therapeutic effect. Co-administration with sensitive CYP3A substrates or CYP3A substrates with a narrow therapeutic index is not recommended. ORKAMBI may substantially decrease hormonal contraceptive exposure, reducing their effectiveness and increasing the incidence of menstruation-associated adverse reactions, e.g., amenorrhea, dysmenorrhea, menorrhagia, menstrual irregular. Hormonal contraceptives, including oral, injectable, transdermal, and implantable, should not be relied upon as an effective method of contraception when co-administered with ORKAMBI
Strong CYP3A Inducers
- Ivacaftor is a substrate of CYP3A4 and CYP3A5 isoenzymes. Use of ORKAMBI with strong CYP3A inducers, such as rifampin, significantly reduces ivacaftor exposure, which may reduce the therapeutic effectiveness of ORKAMBI. Therefore, co‑administration with strong CYP3A inducers is not recommended
Cataracts
- Cases of non-congenital lens opacities have been reported in pediatric patients treated with ORKAMBI and ivacaftor, a component of ORKAMBI. Although other risk factors were present in some cases (such as corticosteroid use and exposure to radiation), a possible risk attributable to ivacaftor cannot be excluded. Baseline and follow-up ophthalmological examinations are recommended in pediatric patients initiating treatment with ORKAMBI
ADVERSE REACTIONS
Serious Adverse Reactions
- Serious adverse reactions, whether considered drug-related or not by the investigators, that occurred more frequently in patients treated with ORKAMBI included pneumonia, hemoptysis, cough, increased blood creatine phosphokinase, and transaminase elevations. These occurred in 1% or less of patients
Most Common Adverse Reactions
- The most common adverse reactions in patients age 12 years and older in Phase 3 trials (Trials 1 and 2) occurring in ≥5% of patients treated with ORKAMBI (N=369) vs placebo (N=370) and at a rate higher than placebo were dyspnea, nasopharyngitis, nausea, diarrhea, upper respiratory tract infection, fatigue, respiration abnormal, blood creatine phosphokinase increased, rash, flatulence, rhinorrhea, and influenza
- The safety profile in patients age 6 through 11 years from an open-label trial (Trial 3; N=58) and a placebo-controlled trial (Trial 4; patients treated with ORKAMBI, N=103 vs placebo, N=101) was similar to that observed in Trials 1 and 2. Additional common adverse reactions were reported in Trial 4, but were not reported in Trials 1 and 2. The adverse reactions in Trial 4 that occurred in ≥5% of patients treated with ORKAMBI with an incidence of ≥3% higher than placebo included: productive cough, nasal congestion, headache, abdominal pain upper, and sputum increased
- The safety profile in patients age 2 through 5 years from an open-label trial (Trial 6; N=60) was similar to that in patients aged 6 years and older. The safety profile in patients age 1 through 2 years from an open-label trial (Trial 7; N=46) was similar to that in patients aged 2 years and older
USE IN SPECIFIC POPULATIONS
Pediatric Use
- The safety and effectiveness of ORKAMBI in patients with CF younger than 1 year of age have not been established
Click here to access full Prescribing Information.
References:
1. ORKAMBI [prescribing information]. Boston, MA: Vertex Pharmaceuticals Incorporated; August 2023. 2. Data on file. Vertex Pharmaceuticals Incorporated. Boston. MA. VXR-HQ-88-00208; 2018. 3. UC Davis Children’s Hospital. (2014, June) How to help your child take medicine. https://health.ucdavis.edu/children/patient-education/How-to-Help-a-Child-take-their-Medicine.


